NervGen Pharma is developing drug treatments that allow the central nervous system to regenerate itself. The company recently demonstrated - for the first time ever -functionally meaningful improvement of motor-recovery in people who have been chronically living with paralysis due to spinal-cord injury for years in a recent Ph1b / 2a trial.
Unlocking potential
We follow a rigorous scientific diligence process and only invest in companies with the greatest potential to commercialize therapies and impact people's lives.

Axonis Therapeutics is developing medicines for neuron restoration and neuromodulation. Therapies are aimed at enabling neural tissue to resist degeneration, restore healthy electrical balance, and promote neural regeneration. Successful pre-clinical POC studies have been completed, and a clinical trial is planned for 2024 / 25.

EG 427 has developed a platform technology to create targeted gene therapies tailored to specific neuronal populations implicated in major pathologies. The first indication is in neurogenic detrusor overactivity (NDO), a form of spastic bladder common in SCI and neurodegenerative conditions such as multiple sclerosis and Parkinson's with significant impact on a person's quality of life and high risk of secondary complications.

Healx is one of the few AI - based discovery companies that have successfully shown the power of their approach across multiple indications from neuroscience to oncology. Their lead program for neurofibromatosis type 1 (NF1) is already in Ph2, within only 4 years of starting the discovery program. SCI Ventures and Healx have partnered to create a neuro-regeneration program with a focus on chronic SCI.
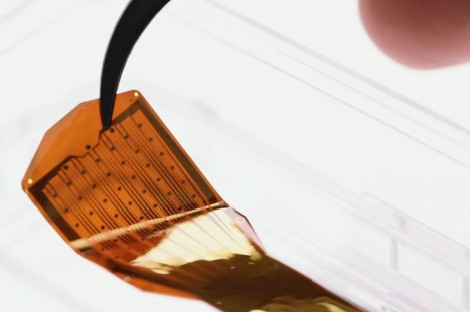
Precision Neuroscience was founded by star neurosurgeon and former Neuralink co-founder Dr. Ben Rapoport to create the next generation of brain-computer interfaces to restore interaction with the environment. The company is seen as a leader among the "Big 6" in BCI, spearheading clinical development with the highest number of patients implanted to date - and the first FDA approval.

Onward is the leader in the spinal cord stimulation space. They focus on targeted, programmed stimulation of the spinal cord to restore movement and other functions, alone or in combination with BCIs for thought-driven movement. Representing some of our most mature technologies, their first FDA approval is on track with the device going to market in 2024.



